COD (Chemical Oxygen Demand) Measurement
This time, I will show you how we measure COD, one of the water quality indicators, at Kendensha.
What is Chemical Oxygen Demand?
Chemical Oxygen Demand (unit : mg/l) is used to measure pollutions caused by organic matters and reducing substances. We choose COD as the indicator for wastewater, water of lakes and the sea, where the water retains. On the other hand, BOD (Biological Oxygen Demand) is used for rivers where the water is constantly flowing and mocro-organisms decompose organic matters.
The oxidising agents used for COD measurement are potassium permanganate and potassium dichromate. Of these, potassium permanganate has been mainly used in Japan (unit: CODmn). Apparently, there are a number of reasons for this, including the fact that when patassoim dichromate is used, the reacted samples contain toxic chromium and mercury, which limit disposal options. However, potassium permanganate is a weaker oxidiser than potassium dichromate and is limited in its ability to detect alcohol and organic acids. This means that it is not ideal for analysing food wastewater. In other countries, potassium dichromate, which has a higher oxidising power, is the standard. Therefore, please be careful when you see COD in Japan. It might mean CODmn, whose value is usually much lower than CODcr.
At Kendensha, we use dichromate method and measure CODcr.
CODcr Measurement
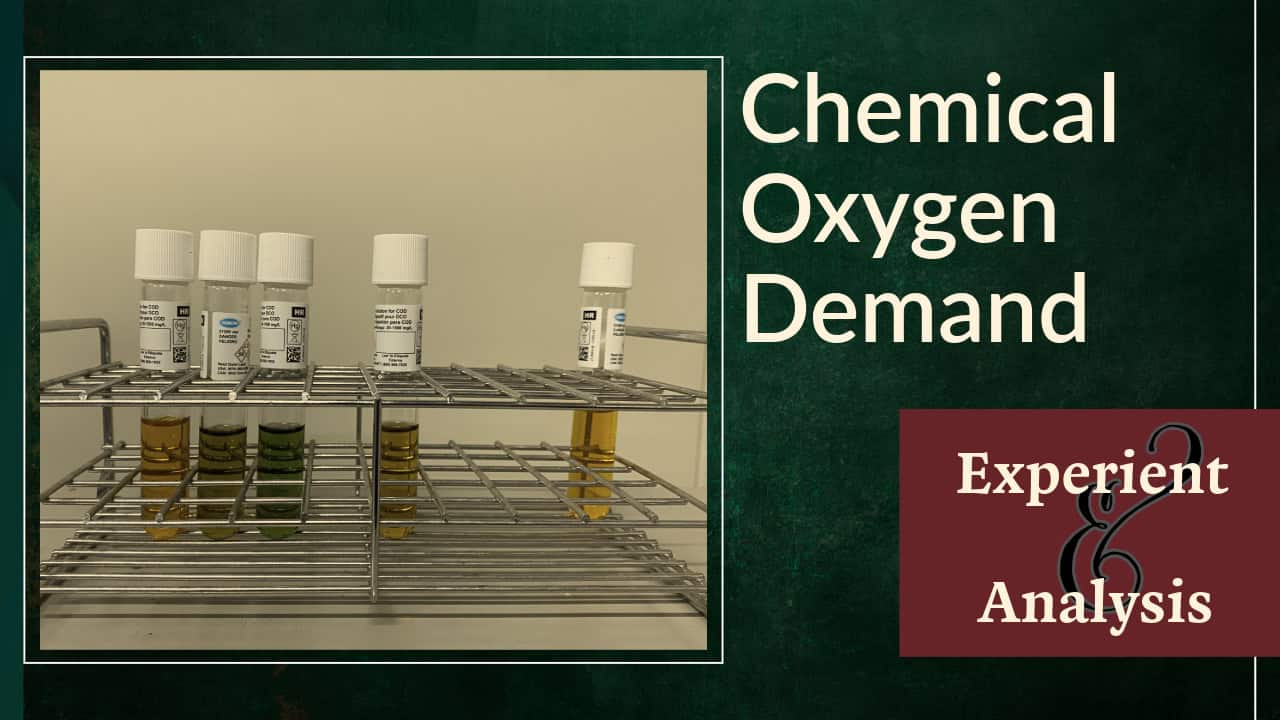
There is the titration method for measuring CODcr, but we use the absorptiometric method for simpler measurement with test vials and devices by Hach company.


Note that the vial becomes very hot.


5. After 2 hours, turn off the reactor and leave to cool for 20 minutes.

It is clear to the eye that orange-red dichromate ions have changed to green chromium ions. The photometer detects the residual yellow Cr6+, from which the amount of oxidised material in the sample is determined.

I hope you will find this a little bit useful.
Internal Links for Water Analysis
- Suspended Solids https://kendensha.com/blog/measuring-ss
- BOD (Biochemical Oxygen Demand) https://kendensha.com/blog/bod-biochemical-oxygen-demand-measurement


